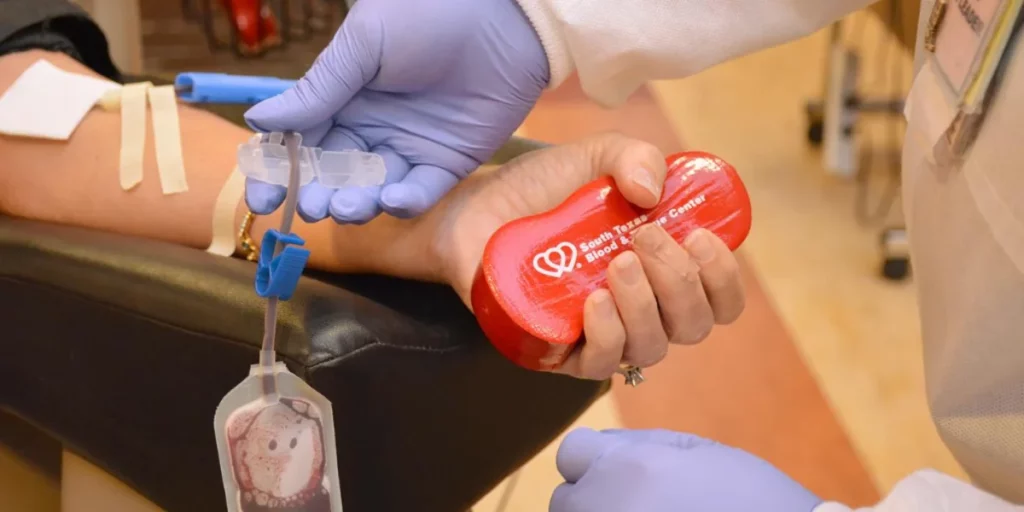
South Texas Blood & Tissue Implements New FDA Guidance for Assessing Blood Donor Eligibility
South Texas Blood & Tissue (STB&T) is implementing new guidance from the U.S. Food and Drug Administration (FDA) for assessing blood donor eligibility using a set of individual risk-based questions to reduce the risk of transfusion-transmitted HIV.