Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on January 14, 2025
For years, the focus in the cell and gene therapy field has been on scientific breakthroughs and regulatory approvals. However, as we’ve reached commercialization for some of these therapies, a new challenge has emerged: getting these therapies to patients at scale.
Here’s the hard truth:
- Many patients and providers are still unaware of the availability of these therapies.
- Even when they are aware, accessing them is far from straightforward.
- Geographic disparities, referral complexities, and logistical hurdles all stand in the way.
- Developing, collecting, shipping, and manufacturing cell therapies requires completely new logistical and supply chain solutions compared to other biologic or chemical-based drug products.
The reality is that innovation means nothing if it doesn’t reach the patient. We need to shift our focus to solving the patient access puzzle to ensure that these therapies don’t just remain scientific achievements but become practical, life-changing treatments.
In this article, I teamed up with a few colleagues, including Vivienne Marshall, PhD, Dr. Rachel Beddard, and Ray Rendon III to explore the key dynamics within this ecosystem, highlight the risks that are impeding patient access, and discuss proven solutions from other areas of healthcare that we can apply to bridge the gap in advanced therapies.
FDA’s Guidance on Decentralized Clinical Trials and Diversity Action Plans
Fortunately, we’re beginning to see regulatory bodies provide guidance to the industry to help address these issues. Decentralized Clinical Trials (DCTs) and Diversity Action Plans, outlined in recent FDA guidance’s, are pivotal frameworks that can help bridge the gap in patient access by making clinical trials more inclusive and accessible to patients in underserved communities.
The FDA’s guidance on Decentralized Clinical Trials (DCTs) encourages clinical trials to move beyond large academic centers and reach patients where they live. This model leverages telemedicine, mobile teams, and local healthcare providers to minimize the travel and logistical burdens that often prevent patients from participating in trials.
In parallel, the FDA’s Diversity Action Plans are focused on improving trial diversity to ensure that advanced therapies are tested in, and accessible to, a broad range of patient populations, particularly those that have historically been underrepresented in clinical research.
Why These Initiatives Matter:
- Decentralized Clinical Trials can help build the experience and infrastructure supporting these therapies that will reduce geographic disparities and make it easier for patients in rural or underserved areas to participate in clinical trials. Participation in clinical trials can also provide access to these therapies for uninsured or underinsured patients.
- Diversity Action Plans ensure that therapies are developed with the needs of diverse populations in mind, improving equity and access once these treatments are commercialized, and including these populations ensures that new treatments are developed that include representative data.
These regulatory frameworks provide a blueprint [for breakthroughs😊] for addressing patient access barriers, but they require innovative thinking and collaboration across the healthcare ecosystem to implement effectively. If these frameworks can take root, it will help solve some logistical challenges limiting patient access today.
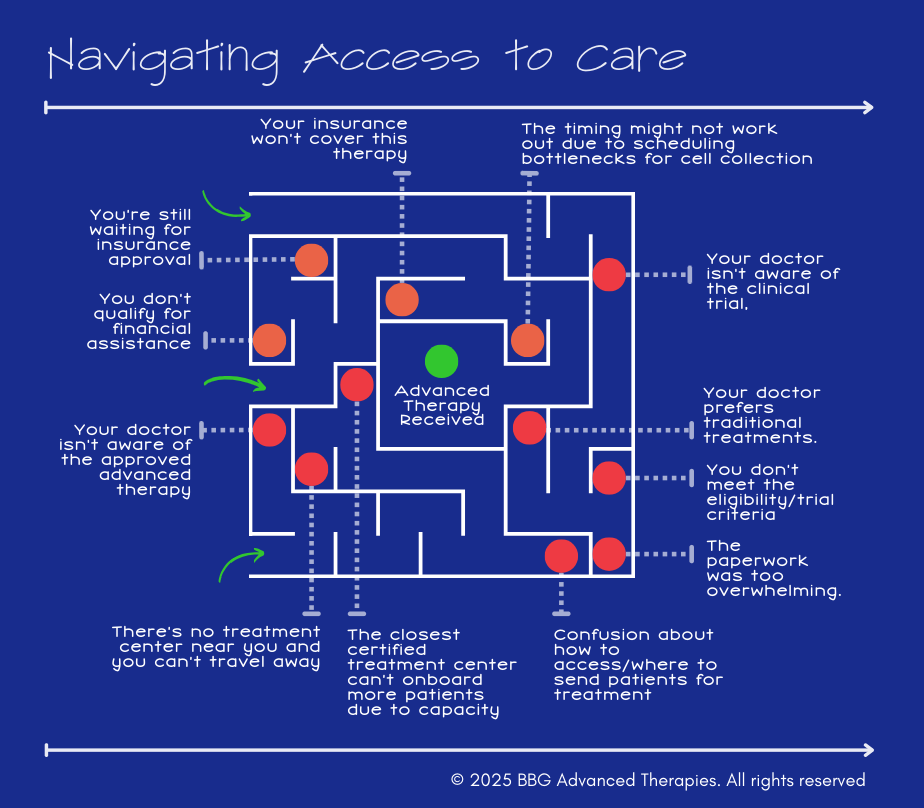
From a Listening Tour: Key Patient Access Barriers
During my listening tours with healthcare providers, patients, and industry leaders, there was a recurring theme: Patient access is a deeply systemic issue that needs more attention. Here are some of the key barriers I heard firsthand:
🔴 1. Gaps in Referral Pathways and Provider Awareness
Many providers don’t know how or when to refer patients for advanced therapies, partly because these treatments are still too new to be widely considered standard of care. For providers in community or non-academic settings, cell-based therapies may not appear in clinical decision-support tools like NCCN guidelines or continuing medical education programs. Without clear referral pathways or formal inclusion in care guidelines, providers may hesitate to recommend unfamiliar therapies, especially those requiring specialized infrastructure or complex logistics, causing delays in patient access.
🔴 2. Geographic Disparities Limit Access
Advanced therapies are typically available only at specialized centers, concentrated in urban areas. Patients in rural or underserved regions face long travel distances and logistical hurdles make accessing these therapies difficult or impossible.
🔴 3. Reimbursement and Financial Barriers
Advanced therapies come with high costs, and reimbursement models haven’t fully adapted to cover these treatments consistently. This creates uncertainty for both patients and providers, leading to delays or denials of care And then there are the associated constraints on a patient’s support circle, oftentimes their family which must travel.

How to Solve Patient Access Barriers: Real-World Solutions from Healthcare
The patient access challenges we face in advanced therapies aren’t unique. Across healthcare, similar barriers have been successfully addressed with innovative solutions. Here’s how we can apply lessons from other areas of healthcare to bridge the gaps in advanced therapies.
While these examples highlight innovative solutions already making a difference in healthcare, they represent just a fraction of what’s possible. The challenges of patient access are multifaceted, requiring diverse strategies across different regions, patient populations, and stages of care. As advanced therapies evolve, so must our approaches.
Leveraging a combination of technology, policy, and collaborative innovation to address gaps that remain. The solutions presented here are starting points, but broader systemic changes and new ideas will be essential to truly scale access for all patients in need.
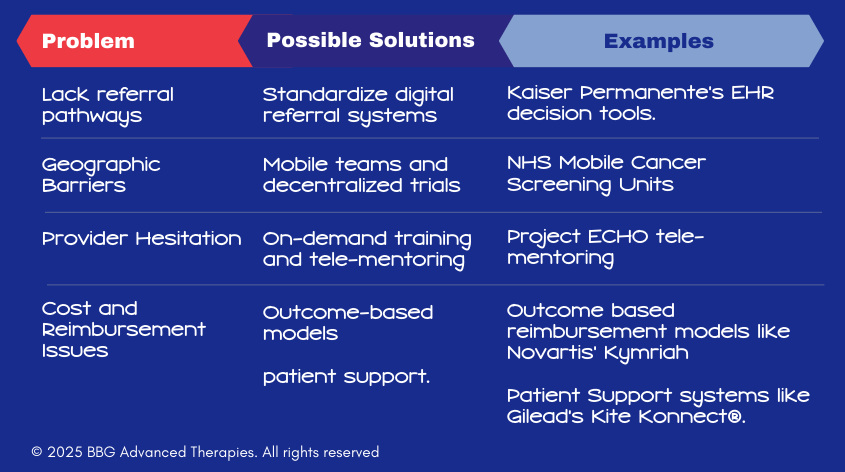
💡 1. Create Clear, Standardized Referral Pathways
The Problem:
Many healthcare providers aren’t sure when or how to refer patients for advanced therapies. This lack of clarity can cause delays, miscommunication, and missed opportunities to offer life-changing treatments.
One Part of the Solution:
Develop referral guidelines and decision trees that make it easy for providers to know exactly which patients are eligible for advanced therapies – and where to send them.
🧩 Real-World Example: Kaiser Permanente’s Referral Optimization
Kaiser Permanente tackled the referral process by implementing electronic referral systems that streamline how patients are sent to specialists. By using decision-support tools embedded in electronic health records (EHRs), primary care providers can easily determine whether a patient qualifies for specialty care, reducing delays in treatment.
How We Can Apply This to Advanced Therapies:
- Build digital referral platforms for cell and gene therapies.
- Provide automated decision tools that guide oncologists through eligibility criteria.
- Ensure patients aren’t stuck waiting for approvals by making the process transparent.
💡 2. Expand Access Through Mobile and Decentralized Solutions
The Problem:
- Access to advanced therapies is often limited to large academic centers, creating geographic disparities. Patients in rural areas must travel long distances to receive care.
One Part of the Solution:
- Use mobile units and decentralized care models to bring advanced therapies closer to patients, reducing travel burdens.
🧩 Real-World Example: The UK’s Mobile Cancer Screening Units
The NHS (National Health Service) in the UK introduced mobile cancer screening vans to reach underserved communities. These units significantly increased early cancer detection rates and improved health equity.
How We Can Apply This to Advanced Therapies:
- Deploy mobile leukapheresis teams to collect patient cells (the therapies starting material) closer to home.
- Partner with regional hospitals to establish satellite cell collection hubs in their community
- Use telemedicine to provide consultations and follow-ups, minimizing travel.
💡 3. Equip Providers with Training and Support
The Problem:
Many oncologists are hesitant to prescribe advanced therapies due to a lack of training or concerns about managing side effects.
One Part of the Solution:
Offer accessible training programs and on-demand support tools to build provider confidence.
🧩 Real-World Example: Project ECHO
Project ECHO uses tele-mentoring to connect rural providers with specialists for complex case reviews. This model has been adopted globally to improve care for chronic and rare diseases.
How We Can Apply This to Advanced Therapies:
- Launch Project ECHO-style programs for cell and gene therapies.
- Develop online training modules on patient selection and side effect management. This would support new providers and practitioners, in currently underserved areas.
- Provide 24/7 consultation services for providers.
- Educate regional hospitals and providers about long-term care and follow-up post-infusion.
💡 4. Innovate Reimbursement & Patient Support Models
The Problem:
- The high cost of advanced therapies creates financial uncertainty for both patients and providers.
One Part of the Solution:
- Develop outcome-based reimbursement models that tie payment to patient outcomes.
🧩 Real-World Example: Novartis’ Kymriah Outcome-Based Payment Model
When Novartis launched Kymriah, they introduced outcome-based reimbursement. If the therapy doesn’t work as expected, payers don’t have to pay the full cost.
How We Can Apply This to Advanced Therapies:
- Advocate for outcome-based contracts.
- Work with payors to create predictable reimbursement pathways.
- Ensure patients aren’t left bearing the financial burden.
Another Part of the Solution:
- Develop patient assistance models that support the patient’s own navigation to access care.
🧩 Real-World Example: Gilead’s Patient Resources Model
Gilead Sciences offers patient support programs designed to help individuals navigate insurance complexities and identify financial assistance options. Programs like Kite Konnect® provide resources tailored to patients undergoing CAR T-cell therapy, ensuring they have the necessary support throughout their treatment journey.
How We Can Apply This to Advanced Therapies:
- Guide patients through the therapy journey and connect them with financial assistance programs.
- Address specific patient needs, such as travel assistance or language translation.
- Partner with patient advocacy groups to provide additional emotional and logistical support.
The Path Forward: Collaboration is Key
Patient access isn’t just a provider problem or a policy issue – it’s our collective challenge. By applying lessons from other areas of healthcare and fostering collaboration across the ecosystem, we can create a future where life-saving therapies are accessible to all.
Working Together: Let’s Bridge the Gap
Addressing patient access barriers in advanced therapies isn’t something any single organization can do alone. It requires cross-sector collaboration across the healthcare ecosystem – from providers and manufacturers to regulators and payors.
Along with the team at BBG Advanced Therapies, I’ve been working on some of these solutions:
- Bring care closer to patients
- Improve trial diversity
We’re committed to aligning our work with these goals to reduce disparities and improve outcomes
If you’re passionate about solving these challenges or have ideas to share, I’d love to hear from you. Let’s collaborate to make patient access a reality. Together, let’s turn innovation into real-world impact.
Join the Conversation
Take the poll to share what you see as the biggest barrier to patient access for advanced therapies in your community. 👉 Do you have any examples?
I’d love to hear your thoughts and ideas. Comment below, share with others to join our community of innovators and advocates and to subscribe to Blueprint for Breakthroughs. Let’s continue this important conversation.
📖 References and Further Reading
FDA Guidance on Decentralized Clinical Trials (DCTs)
FDA Draft Guidance on Diversity Action Plans
Kaiser Permanente Referral Optimization
Project ECHO
Novartis Kymriah Outcome-Based Payment Model
Gilead’s Kite Konnect® Patient Navigation Model



