Since 2009, our apheresis center has helped patients by collecting cells for lifesaving treatments and research. We work closely with biotech and pharmaceutical partners to support clinical trials through drug development, approval and commercialization.
Experts maintain and grow a local registry of more than 35,000 recallable research donors, who are informed about opportunities to participate and willing to be contacted.
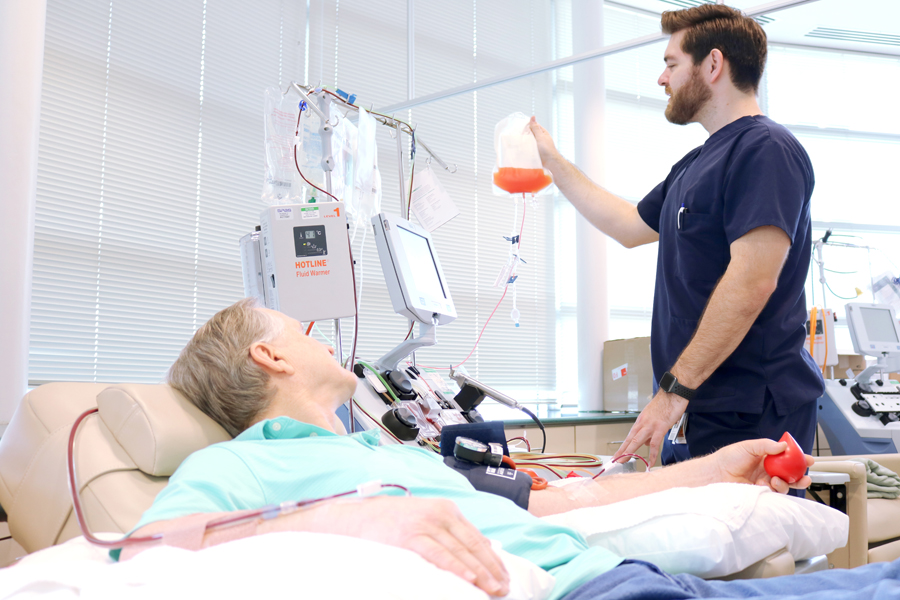
Licensed nurses and physicians work with donors to confirm eligibility to prepare them for collection.
We perform cGMP collections, testing, cryopreservation and shipping within our enterprise Quality Management System.
Our facility houses an onsite AABB-accredited and CLIA-certified testing laboratory compliant with U.S. and multiple international agencies. Our laboratory services include:
Our Research & Development team supports custom assay development.
