
BioBridge Global gives tour to Blood Centers of America
BioBridge Global recently gave a tour of subsidiary GenCure to Blood Centers of America Advanced Therapies Network.

BioBridge Global recently gave a tour of subsidiary GenCure to Blood Centers of America Advanced Therapies Network.

Becky Cap to lead business development efforts

Veteran scientist has led expansion of BioBridge Global’s research and development capabilities

REPROCELL Inc. and BioBridge Global have signed a Memorandum of Understanding to enhance their strategic collaborative relationship
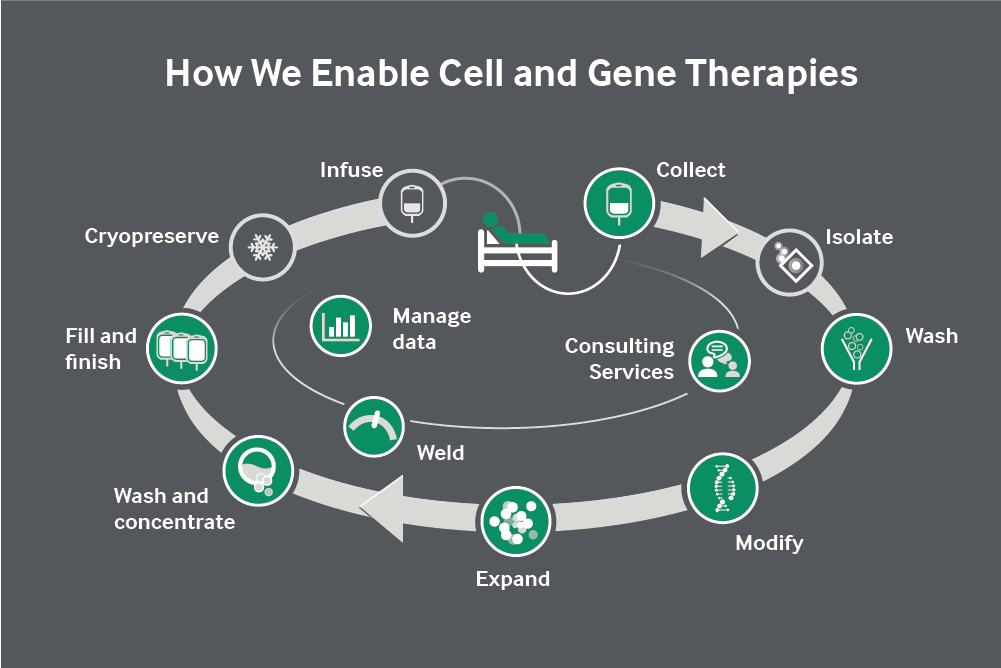
Unique collaboration begins to link the CGT supply chain from end to end to tackle hard scale-up and quality challenges across the industry

Rigorous review process leads to accreditation for peripheral blood collection under high quality care for patients
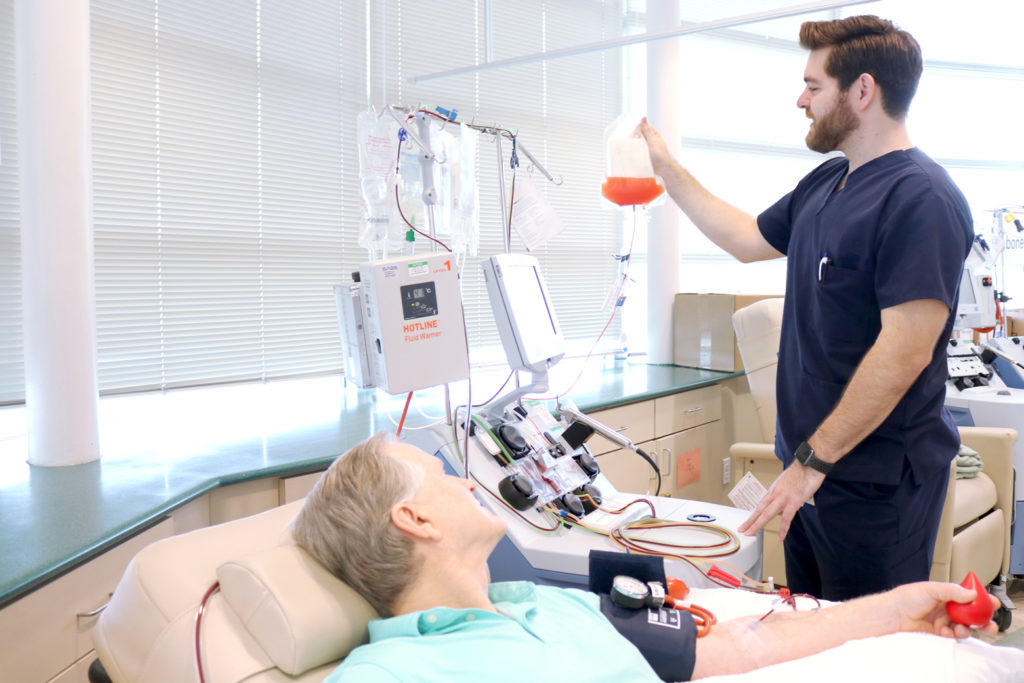
Center for Advanced Therapies is part of the ever-expanding field of cellular and immune therapies, assisting with various phases of research into therapies to cure chronic, debilitating and degenerative diseases.

Stem cells from cord tissue to be used to develop new cellular therapies

The team at GenCure biomanufacturing is becoming a part of the rapidly growing community on San Antonio’s East Side.

Partnership to develop new cryopreservation systems for the advancement of human blood services and advanced cellular therapies

From new assays to new ways of looking at donations, BBG R&D is making an impact.

This whitepaper goes through five testing strategies that QualTex Laboratories use to help customers in the cell and cell-based therapy industry.