
Emergency preparedness agency recognizes South Texas Blood & Tissue
South Texas Blood & Tissue has been presented the 2023 Partnership Recognition Award by the Texas Division of Emergency Management.

South Texas Blood & Tissue has been presented the 2023 Partnership Recognition Award by the Texas Division of Emergency Management.

BioBridge Global and South Texas Blood & Tissue announced today that Audra Taylor, SBB, is joining the organization as Executive Director for Blood Operations.

Becky Cap to lead business development efforts

Veteran scientist has led expansion of BioBridge Global’s research and development capabilities

Blood centers across the United States, including South Texas Blood & Tissue, are celebrating the one-year anniversary of the nation’s first emergency blood reserve program.

South Texas Blood & Tissue has partnered with the National Institutes of Health’s All of Us Research Program, to recruit and engage participants in one of the nation’s largest and most diverse health information databases.

Bulverde-Spring Branch area location to offer blood donor center in one of America’s fastest-growing areas
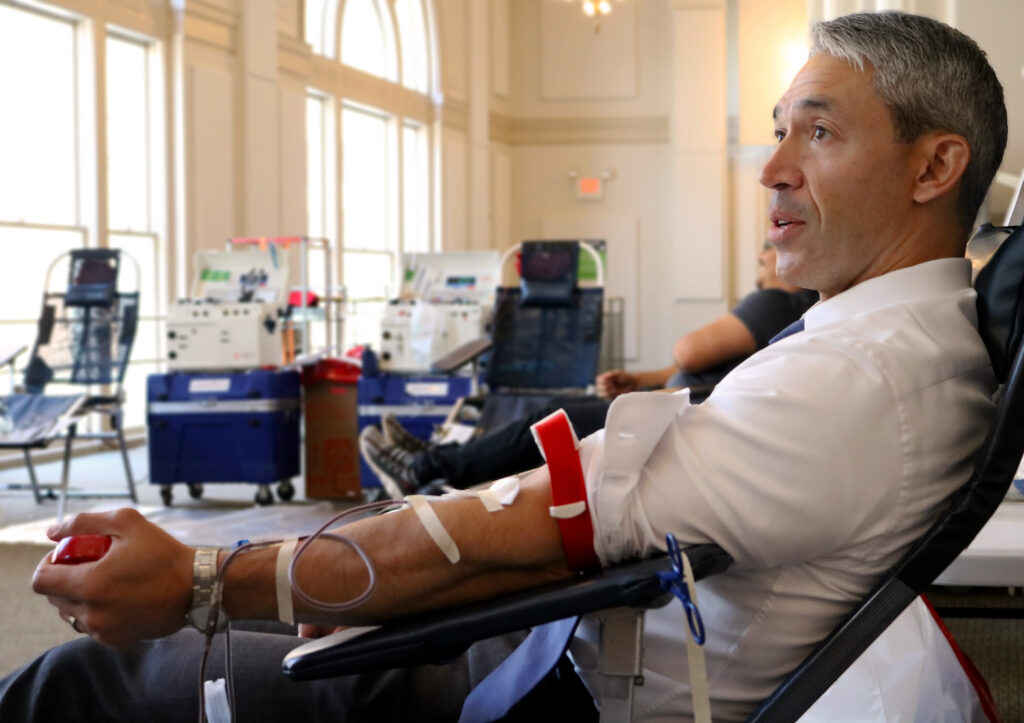
To bring awareness to the summer season when blood donations slow down, the City of San Antonio is hosting blood drives for city and county employees.

Adrienne has taken on multiple challenges throughout her career, stretching her capabilities and her knowledge of her interests while thinking of herself a lifelong learner and adventurer.

Surprise guests, heartfelt thanks and a really big card helped the organization commemorate the retirement of Elizabeth Waltman.

Rigorous review process leads to accreditation for peripheral blood collection under high quality care for patients

During the recent send-off event for Elizabeth Waltman, she received a special honor: The THOR sword.