
Advanced Therapy New Focus for BioBridge Global
Becky Cap to lead business development efforts

Becky Cap to lead business development efforts

Veteran scientist has led expansion of BioBridge Global’s research and development capabilities

REPROCELL Inc. and BioBridge Global have signed a Memorandum of Understanding to enhance their strategic collaborative relationship
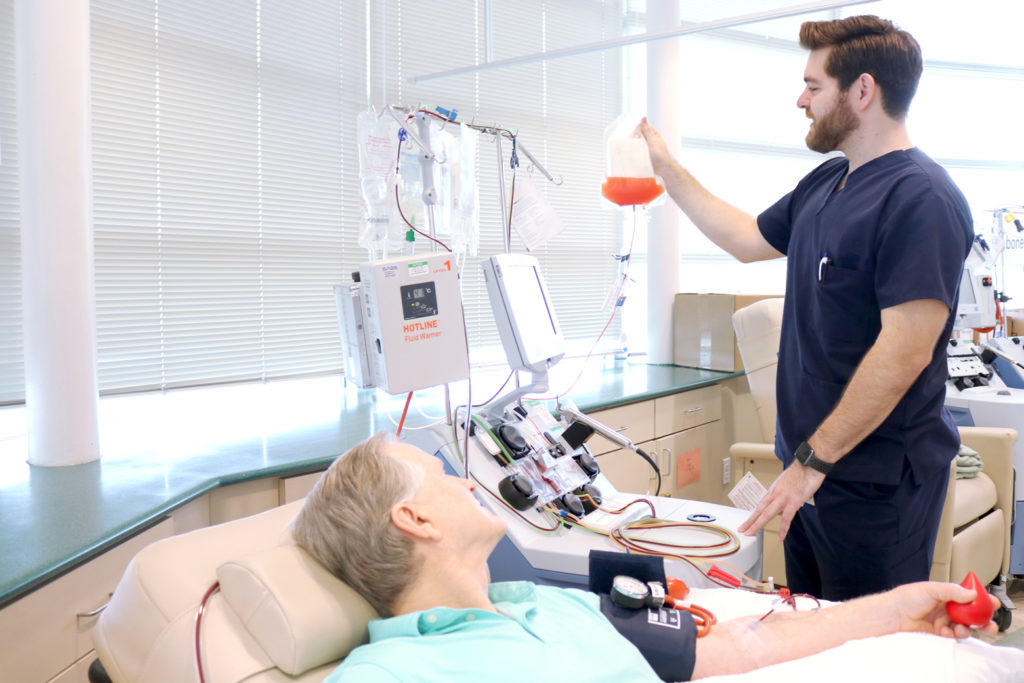
Center for Advanced Therapies is part of the ever-expanding field of cellular and immune therapies, assisting with various phases of research into therapies to cure chronic, debilitating and degenerative diseases.

Stem cells from cord tissue to be used to develop new cellular therapies
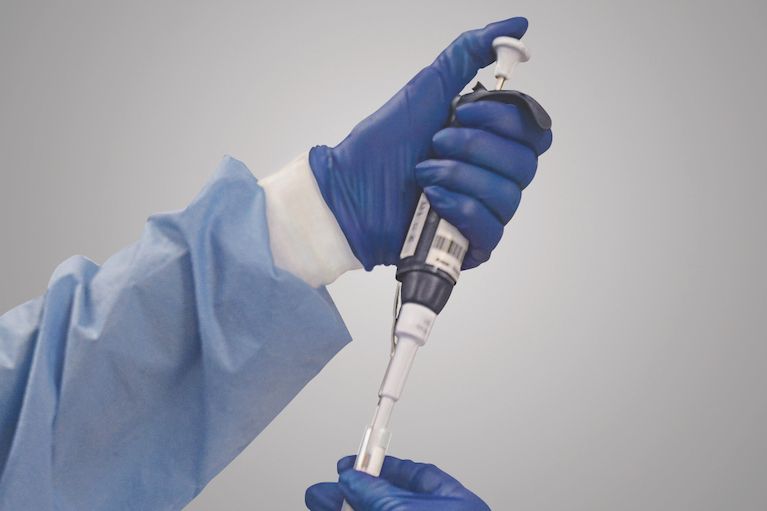
Lab to support researchers developing new treatments for global healthcare market including cellular therapy QualTex Laboratories, a subsidiary of the San Antonio-based nonprofit BioBridge Global,
Partners GenCure, Sentien, & RoosterBio will develop potency assay for mesenchymal stromal cells The federal Medical Technology Enterprise Consortium (MTEC) has identified the need for

COVID-19 testing facility based at BioBridge Global receives $1 million boost Getting the Community Labs COVID-19 testing program up and running in just three months is a