Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on August 26, 2025
How Does Our Industry Deliver Cell and Gene Therapies Today?
Today, access to cell and gene therapies is primarily delivered through inpatient stays at academic research hospitals, governed by complex Risk Evaluation and Mitigation Strategies (REMS) requirements and specialty site certifications. These centers were essential in the early days — and they remain critical for emerging indications and first-in-human modalities, equipped with the infrastructure, expertise, and oversight to manage fragile products in patients who had exhausted every other option.
But this highly centralized, hospital-based model is not built for scale.
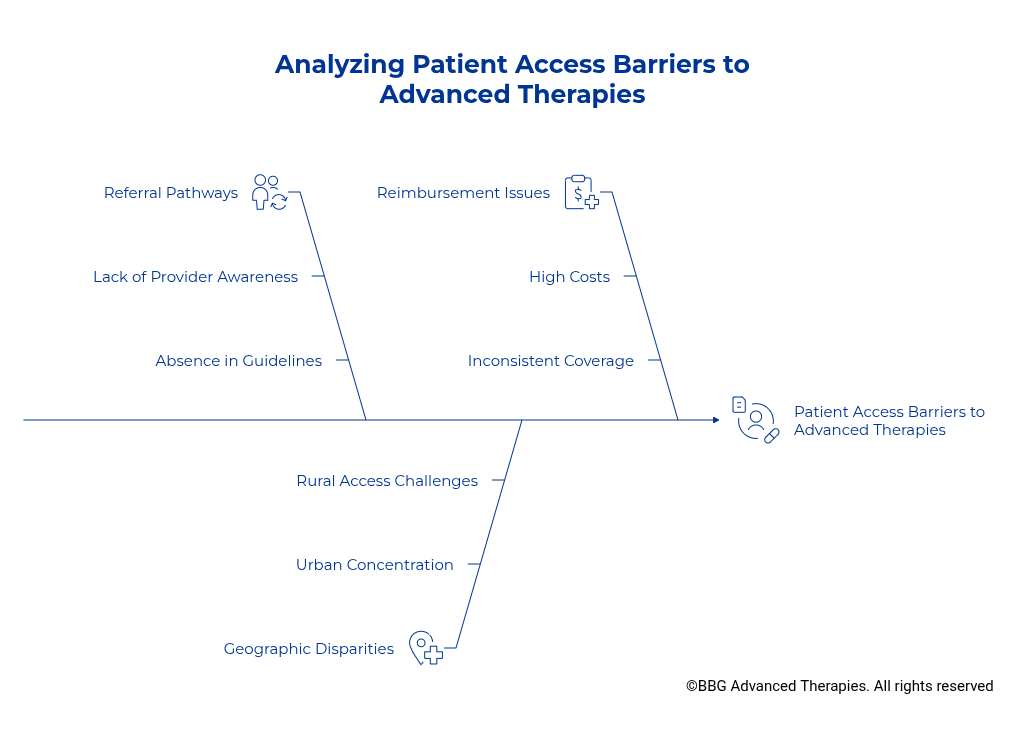
Why This Model Falls Short
Cell and gene therapies are no longer limited to late-stage oncology. They are rapidly becoming first- and second-line treatments in blood cancers, as well as advancing into autoimmune disorders, genetic diseases, and rare conditions. Tomorrow, they will reach much broader patient populations.
Yet, our current delivery system still locks access largely inside the walls of specialty hospitals. This creates unnecessary bottlenecks that:
- Overburden elite academic centers with procedures that could safely shift elsewhere.
- Tie up scarce resources needed for sicker patients and groundbreaking research programs.
- Reinforce inequities by limiting availability to a handful of geographies.
The truth is simple: the system we have today cannot scale to meet what’s coming.
The Access Gap, in Plain Sight
I see this vividly in my own community of San Antonio, Texas. Very few local clinicians or oncologists even know these therapies, beyond bone marrow transplants, which have been widely available for over 20 years and a practice of medicine since the 1960s, exist despite multiple FDA-approved, commercially available therapies. Patients here should have the same access as those in Pennsylvania, California, or Houston. Instead, they are left behind, trapped by the artificial limits of a delivery system that has failed to expand with the science.
By limiting delivery to a narrow circle of major institutions, we send an unintended message: that most clinicians aren’t capable of managing this care. But that’s simply not true. Providers across the country routinely handle new modalities, manage complex side effects, and adapt to evolving standards. Underestimating their capacity undervalues the very system we rely on to scale.
Outpatient vs. Inpatient: The Next Chapter in Access
Historically, most cell and gene therapies — including CAR T — were administered as hospital inpatients due to safety concerns. However, more protocols are now enabling outpatient administration, driven by improved patient selection, better toxicity management, and new monitoring technology.
- Comparative studies show response rates and safety profiles are similar between inpatient and outpatient delivery. (NIH Research Study)
- Outpatient care reduces costs by up to 40%, eases patient burden, and improves quality of life — allowing recovery at home surrounded by family.(US-Pharmacist)
- Nearly 70% of patients in recent observational cohorts received outpatient CAR T cell therapy, and 25% of those never required any hospital admission at all. (Hematology)
- With comprehensive protocols and real-time monitoring, even centers new to CAR T cell therapy can safely manage outpatient administration, extending access well beyond academic hubs.(ASCO)
This shift gives us a unique opportunity to carefully and intentionally rewrite the playbook on access: with deliberate strategies, outpatient models can decentralize delivery, close care deserts, and finally bring equity to advanced therapies.
Reimagining the Access Model
To scale access, we must reimagine the entire delivery system:
- Collection: Use dedicated specialized teams — including mobile GMP-compliant platforms — to bring leukapheresis directly to the patient, wherever they are.
- Clinical Sites: Rather than replicating costly hospital certification everywhere, allow qualified community clinics to receive and administer therapies, provided they have protocols for monitoring and managing side effects.
- System Support: Regulators, payers, and industry must align policies, reimbursement, and accreditation with this distributed model.
This is not about lowering standards. It’s about rethinking standards to enable safe, scalable delivery.

Leukapheresis & Apheresis Strategies
Rethinking how cell and gene therapies are delivered starts with identifying the practical bottlenecks and gateways that shape patient access. Nowhere is this more evident than in the realm of leukapheresis (also called apheresis) — the foundational first step for almost every autologous cell therapy protocol. Historically, the collection of cellular starting material has been tethered to major academic hospitals or a small number of fixed apheresis sites, unintentionally recreating the same geographic silos and capacity strains that limit downstream treatment. To drive real change, we must also consider the physical infrastructure and deployment of leukapheresis services themselves since by expanding beyond frameworks, we create a platform to activate more clinical sites, since the site requirements change, thus we can support diverse patient populations, and make scalable, equitable access a practical reality.
Which Leukapheresis Model?
A variety of innovative models now exist to decouple leukapheresis from the confines of the primary clinical site, each with unique strengths, limitations, and clinical use cases. Fixed-site apheresis centers, mobile leukapheresis solutions, and in-hospital bedside teams all serve distinct patient populations and operational scenarios.
- Fixed-site apheresis centers offer stability and high-throughput capacity, best suited for metropolitan regions where patient volume can justify a permanent facility and patients can readily access care close to home.
- Mobile leukapheresis centers shine in geographically expansive regions or where patients are widely distributed and outpatient clinical sites are far apart. By bringing a fully equipped, environmentally controlled unit to community practices, these mobile teams accelerate the activation of new clinical sites, allow expertise and high-end equipment to be efficiently shared across distances, and reduce both logistical and financial barriers to access, especially in rural or underserved areas.(Mobile Leukapheresis Center)
- In-hospital/beside teams remain indispensable for non-ambulatory or acutely ill patients requiring continuous care during collection. These teams offer the flexibility to support specialized patient needs directly within the hospital environment without hospital transfers.
Each model comes with trade-offs in terms of operational complexity, resource allocation, scalability, and cost-effectiveness. An integrated delivery network, leveraging all three approaches, is essential for building robust, equitable access to advanced therapies during this transition in our industry, where there is a mix of inpatient and outpatient protocols and still limited clinical sites for the administration of the final drug.
By tailoring the choice of leukapheresis delivery model to the geography, patient mobility, and site experience, the industry can optimize both quality and reach—unlocking a future where site activation is no longer a limiting step but a catalyst for delivering cell and gene therapies everywhere they are needed
What About U.S. Policy — Is There Support for This Approach?
Yes. On several important fronts, the regulatory frameworks are evolving to recognize that therapies can be delivered more widely. Confusion persists around payor models, but in reality, the current payor framework already supports contracted, decentralized collection.
FDA – REMS Removal Signals Broader Access
On June 27, 2025, the FDA eliminated REMS (Risk Evaluation and Mitigation Strategies) requirements for all currently approved BCMA- and CD19-directed CAR‑T cell therapies, including Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, and Yescarta (FDA Source)
What this means:
- No more need for special site certification or on-site access to tocilizumab.
- Safety can be managed via product labeling — including boxed warnings and Medication Guides
- Disease monitoring periods (e.g., post-infusion restrictions like driving limitations or staying near a center) have been significantly shortened for some products
This is a clear shift toward trusting certified providers beyond academic hubs to administer these therapies safely — and a strong signal that regulatory frameworks can evolve to unlock new places of access.
Centers for Medicare & Medicaid Services (CMS)
One of the biggest myths in this field is that reimbursement requires hospital delivery. That is simply not true.
As I outlined in my recent CMS reimbursement analysis, leukapheresis is not the revenue driver within bundled payments. Hospitals do not need to perform it themselves to sustain their payment structure.That means compliant, contracted apheresis centers — including mobile or outpatient GMP-ready platforms — can collect cells without disrupting the hospital’s reimbursement. In fact, by moving this non-revenue-producing step outside of the hospital, we reduce burden on institutions, streamline patient care, and allow hospitals to focus on what does drive value: treatment and patient management.
Next Steps?
If these insights have resonated with your organization or sparked ideas about expanding advanced therapy access, let’s connect. Whether you’re navigating logistical barriers, planning for site activation, or seeking a deeper understanding of how flexible cell collection models can accelerate your work, I’m here to help. Contact me directly to discuss how these innovative solutions can move your program—and patient access—forward.



