A Blueprint for Early-Stage Success
Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on February 11, 2025
The biotech industry enters 2025 amid a paradox: while global investment is projected to grow from $483 billion in 2024 to $546 billion (Qubit Capital), early-stage companies face unprecedented pressure. Venture capital is increasingly funneled into late-stage programs with clear commercialization potential, leaving preclinical innovators competing for shrinking resources. Simultaneously, NIH budget freezes and a cautious IPO market amplify the stakes for early-stage biotechs.
In this high-risk environment, missteps during preclinical development, whether due to fragmented workflows, scalability gaps, or regulatory misalignment, can derail progress and deter investors. Yet for companies willing to adopt a strategic approach, the challenges also present an opportunity to differentiate themselves.
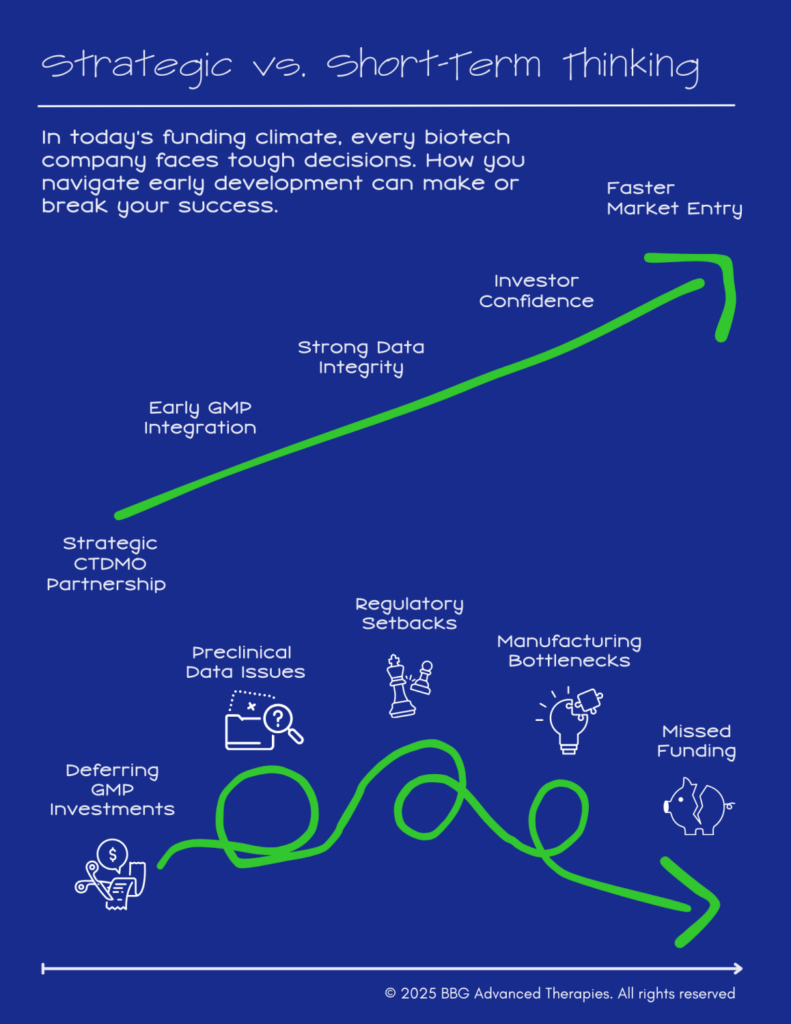
Turning Challenges into Opportunities: How Smart Companies Surge Ahead
The most successful organizations will be those that prioritize quality, scalability, operational efficiency, and strategic partnerships from the start. By avoiding shortcuts, embracing creative funding models, and aligning with experts who understand the complexities of scaling therapies, early-stage biotechs can transform financial headwinds into tailwinds.
At the heart of this strategy lies collaboration. Partnering with experienced teams ensures not only robust preclinical data and regulatory compliance but also a shared commitment to navigating challenges proactively. Through open communication and tailored solutions, even in a constrained funding climate, biotechs can safeguard their programs and accelerate toward commercialization.
Creative Funding Models for Early-Stage Success
In today’s challenging funding landscape, early-stage biotechs may need to explore alternative strategies to stay competitive:
- Royalty Financing: Obtain upfront capital in exchange for a percentage of future product revenues, which can be ideal for companies nearing commercialization. From 2012 to 2023, $26.4 billion in royalty deals were executed.
- Venture Loans: Opt for fixed-term loans with regular repayments, offering flexible growth capital while maintaining full ownership. These methods are gaining momentum with lenders offering loans up to $100 million for clinical-stage programs.
- Public-Private Partnerships: Reduce costs and risks by collaborating with public organizations. Pfizer’s ARDAT initiative, with 30+ partners, is a prime example, cutting gene therapy development costs.
- Milestone-Based Funding: Secure funds in stages tied to development milestones, mitigating investor risk and showcasing consistent progress to attract continued support.
- Licensing & Co-Development Deals: Partner with larger firms for upfront capital, shared resources, and technical expertise while retaining control over your technology. These collaborations can fast-track market entry and boost investor confidence.
By leveraging these creative strategies, early-stage biotechs can continue advancing, even in tough financial climates.
Investors expect developers, in any case, to meet strategic milestones on a clear timeline, and having experienced, high-quality partners significantly increases the probability of success. BBG Advanced Therapies stands out by offering comprehensive, end-to-end solutions tailored to meet each customer’s unique needs. From starting materials, and analytical and process development, to Good Manufacturing Practice (GMP) and regulatory support. Unlike larger Contract Testing, Development, and Manufacturing Organizations (CTDMO or CDMO), BBG Advanced Therapies brings flexibility and adaptability, ensuring faster, more efficient progress while building investor confidence through consistent, high-quality execution.
Preclinical Data Integrity
Robust, properly documented preclinical data is essential for accelerating regulatory approvals and securing the funding needed to advance therapies. By producing and testing preclinical batches that meet specifications aligned with first-in-human studies, companies ensure consistency, build trust, and increase confidence among both investors and regulators.
BBG Advanced Therapies utilizes a state-of-the-art Electronic Batch Production Record (eBR) system that enhances data capture, improves data integrity, and provides deeper insights from production data. This technology not only streamlines processes and eliminates costly rework but also ensures the highest level of accuracy and traceability.
In contrast, many CTDMOs or CDMOs still rely on cumbersome manual record-keeping or basic tools like fillable PDFs, which limit data usability and hinder efficient analysis. By investing in advanced systems from the start, BBG Advanced Therapies helps de-risk development and accelerate the path to regulatory success.
How the Right Partner Can Accelerate Your Success
In a crowded field, reliable data and a clear path to commercialization will separate leaders from those scrambling to recover from early mistakes. This is why companies that align with experienced, forward-thinking collaborators will not only navigate today’s challenges but outpace competitors who cut corners or delay critical investments.
An experienced Contract Testing, Development, and Manufacturing Organization (CTDMO) with access to critical starting materials can provide the expertise needed to avoid common pitfalls while accelerating development timelines.
BBG Advanced Therapies offers comprehensive support, from starting materials, process and analytical development, to GMP manufacturing, cell therapy testing, cryopreservation, and logistics – all backed by cutting-edge R&D expertise and a fully centralized Quality Management System (QMS) across all programs. Every service is designed to reduce risk and maximize efficiency:
- Critical-to-Quality (CTQ) Safeguards: Identifying and protecting key attributes early ensures product integrity and investor confidence.
- Streamlined Processes: By focusing resources where they matter most, we minimize costs and prevent unnecessary delays.
- Preclinical Data Integrity: Producing and testing preclinical batches to specifications aligned with first-in-human studies ensures consistency, builds trust, and accelerates acceptance by both investors and regulators.
By integrating risk management with operational efficiency, you’ll not only avoid costly rework but also establish a solid foundation for clinical success.
Your Roadmap to Success
The decisions you make today will define your success tomorrow. With BBG Advanced Therapies as your partner, you gain more than a service provider…you gain a strategic ally dedicated to maximizing your growth potential. When your provider ensures your program is built on a foundation of quality, scalability, and compliance, it enables you to advance confidently, even in today’s challenging funding climate.
Let’s Discuss Your Path Forward
Reach out to me, or visit www.bbgat.org, to see how BBG Advanced Therapies can help you maintain momentum and turn today’s funding challenges into opportunities for growth and leadership in the biotech space.



