Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on February 25, 2025
In biotech, every decision made in early development can ripple through the entire lifecycle of a therapy. One often-overlooked decision? Data maturity of batch production records – and when to implement an electronic batch records (eBR) system.
Many assume paper-based or manually digitized records will suffice through preclinical work and even into clinical trials. However, this short-term savings mindset often leads to costly rework, delayed regulatory filings, and data integrity challenges that ultimately slow a therapy’s path to patients.
At BBG Advanced Therapies, we’ve taken a different approach. By integrating MasterControl, a fully digital eBR platform, and combining our best practices and our clients specific process needs from the start, we’ve built our digital infrastructure seamlessly incorporating client processes with our own digitized workflow. Thishelps our clients avoid costly documentation and scalability pitfalls while enhancing long-term scalability and data-driven insights.
The decision to adopt early data maturity matters more than ever, and in the following article, we will discuss how early eBR adoption can become a competitive advantage in today’s biotech landscape. In future editions, we will also dive into additional benefits, long term of these digital assets, including the opportunity to leverage vast amounts of data to draw insights from and contribute meaningfully to risk and cost reductions.
Building Data Integrity from Day One
Regulators and investors alike scrutinize preclinical data for consistency and reliability. Paper-based records or fillable PDFs, still incredibly common in early-stage biotech manufacturing, introduce risks of transcription errors, incomplete or non-contemporaneous documentation, and data loss.
With an electronic batch record (eBR) system:
- ✅ Data can be captured directly from source, minimizing the reliance on manually entries.
- 🔒 Built-in audit trails and real-time monitoring support regulatory requirements like FDA 21 CFR Part 11.
- 🚩 Atypical entries are flagged and available for review instantaneously.
- ⏱️ Batch records are immediately available for review. This means no waiting for manual reconciliation or handoffs.
- ⌨️ Data inputs are only required for activities observed or performed as the logic-based and automated capabilities can be leveraged to perform calculations and prior manufacturing step dependencies.
As Emmanuel Casasola, BioBridge Global’s Head of Global Quality & Regulatory, noted:
“When preclinical and clinical production data demonstrate clear integrity and traceability, it becomes easier to build trust with regulators and potential funding partners. Investors aren’t just looking for great science anymore. They want to see that the development process is robust, scalable, and ready for clinical and commercial execution.”
The Hidden Costs of Deferring eBR
When teams delay eBR implementation until Phase 3 or commercialization, they often encounter costly setbacks:
- 🛠️ Rework and Data Reconciliation Manual records from preclinical and early clinical stages rarely meet regulatory standards for commercial filings.
- ⚙️ Process Gaps During Scale-Up What works in small-scale development might not translate to clinical or commercial batches. Without consistent, traceable records from the start, teams often face unanticipated variability during scale-up.
- ⏳ Slower Regulatory Approvals IND, BLA, and marketing applications require complete, auditable data trails. Delays in generating these records can extend approval timelines and erode investors’ patience.
- 🧐 Investor Skepticism In today’s environment, potential investors want more than compelling science; they expect operational readiness. Biotechs that demonstrate early GMP alignment and transparent process data stand out as safer bets.
BioBridge Global’s VP, Technology & Innovations, Anand Srinivasan, Ph.D. remarked:
“People may know about the concept of eBR, but there’s often an intimidation factor around the cost and timeline. But the reality is that starting early makes everything downstream more predictable and less expensive.”
How Early Adoption Pays Off
Biotech development success relies on the ability to consistently tell a data-driven story that regulators, investors, and partners can trust. Reliable, accessible manufacturing data supports that narrative.
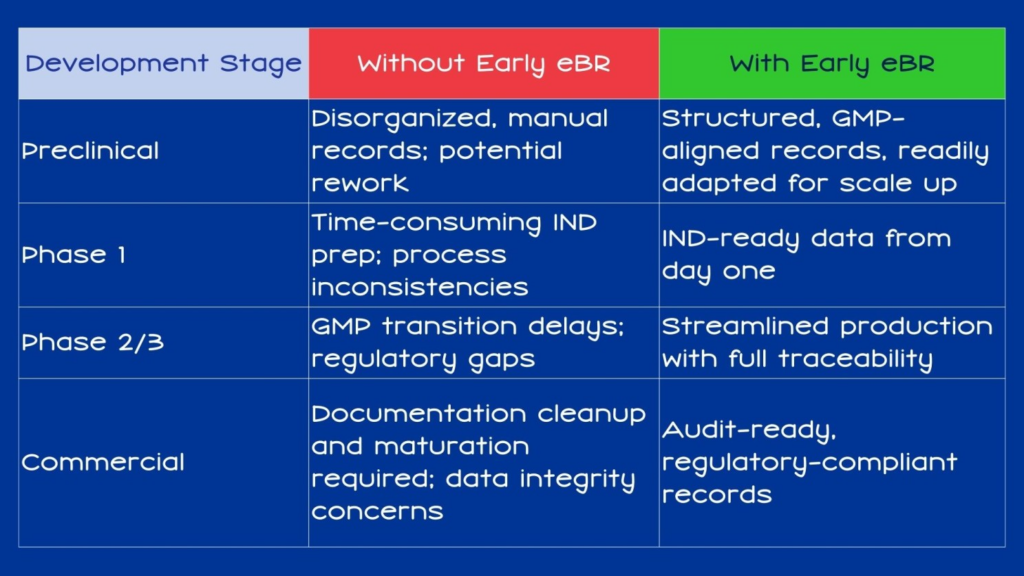
With early eBR adoption, companies benefit from:
- ✅ Improved Data Integrity: Standardized formats reduce the risk of transcription errors and data loss.
- 🔄 Seamless Process Evolution: Records evolve alongside processes, ensuring consistent traceability from preclinical work to commercial production.
- 🔬 Process Development Transparency: Records can clearly link process steps to critical process parameters and quality attributes.
- Compliance: Audit trails are automatically created, with every process change captured and documented.
- 📊 Meta Data Analytics: Digital data can provide insights to manufacturing processes that can be relied upon as scale up occurs throughout the commercialization process that investors and regulators can trust…. The Importance of a Platform Designed for the Complexity of Advanced Therapies
Cell and gene therapy manufacturing introduces unique challenges that demand a purpose-built eBR approach. The variability in starting materials, personalized production timelines, and complex supply chain logistics all require more than a basic documentation tool.
That’s why BBG Advanced Therapies didn’t just add eBR to our manufacturing processes – we built our infrastructure with these systems in mind from the start. We use a fully integrated eBR platform that connects batch records to our entire quality management system, including direct connection to all controlled documents, training records, equipment, material, quality events, change control, and more.
This approach ensures every aspect of production is tracked, verified, and easily auditable, with data integrity principles built into the process from day one.
Building vs. Partnering: What’s the Right Move?
Once a company recognizes the value of early eBR adoption, the next decision is whether to build the infrastructure themselves or outsource to an experienced Contract Testing, Development, and Manufacturing Organization (CTDMO). Both approaches have merit, but the best choice often depends on the company’s resources, timeline, and growth plans.
Building In-House
Biotechs that plan to internalize manufacturing may choose to implement an eBR system internally. This route can offer long-term control but requires:
- 💰 Substantial upfront investment in software, hardware, and personnel
- 🛡️ Ongoing maintenance and compliance oversight
- ⏳ Time-consuming process development for each new product
For early-stage companies already facing financial pressures, these costs can stretch timelines and limit other R&D investments.
Partnering with an experienced and well-equipped CTDMO
Alternatively, partnering with a CTDMO, like BBG Advanced Therapies that already has GMP-compliant eBR capabilities offers immediate access to:
- 📜 21 CFR Part 11-Compliant Systems: Ready for regulatory review from the start
- ✏️ Customizable Batch Records: Tailored to the needs of each process while maintaining consistent data structures
- 🤝 Seamless Quality Integration: Data linked to enterprise systems for material tracking, equipment control, and training oversight
- 🖥️ Realtime Monitoring Capabilities: Remote view level assess and notifications for partners allow for streamlined updates and collaboration
This approach delivers all the benefits of eBR adoption without the overhead of building and maintaining the system independently.
“The advantage of working with BBG Advanced Therapies is that you don’t have to shoulder the investment burden upfront,” explains Joe Higdon, Director, Quality & Compliance for BBG Advanced Therapies. “We’ve already built the infrastructure and expertise so that early-stage programs can access these benefits for tailor-made processes without reworking their data down the road.”
BBG Advanced Therapies encourages customers to adopt eBR capabilities early, even when they are engaged in process development (PD). By allowing the PD team to have access and capability to build out eBRs, they can ensure that the process flow and documentation are built in a coordinated effort that allows for a robust and repeatable manufacturing process. While manufacturing collaborating with Quality and Manufacturing personnel to ensure compliance and readiness.
Dr. Srinivasan noted:
“With the advent of AI and increasing implementation of machine learning, deep learning, and computer vision in the development and manufacturing process, the attempt to digitize is pivotal. The widespread adaptation of machine learning tools combined with automated process analytical technologies will enable predictive analytics to garner deeper insights during the development, technology transfer, and manufacturing of advanced therapies. Manual data entry is not feasible for the large dataset collected during product development lifecycle, and selective digitization of data would result in loss of holistic insight.”
Why Early Data Maturity Matters Now
In a funding environment where every milestone is scrutinized, operational maturity can make or break an investor pitch.
Early-stage biotechs that integrate eBR into their development workflows can:
- ✅ Prove Process Maturity: Demonstrate readiness for clinical trials with detailed, reliable records
- 💰 Optimize Resources: Reduce costly rework and streamline regulatory interactions
- 📈 Build Market Advantage: Position themselves as high-quality, investment-ready programs in a competitive landscape
- 🤝 Enhanced Technology Transfer: Through the consolidation of data and information, eBRs allow for more efficient and effective document and knowledge transfer to potential receiving units.
The Path Forward: Build with the End in Mind
At BBG Advanced Therapies, we’ve collaborated with MasterControl to support lean, efficient implementation – even for companies with limited resources. Our organization is poised to help therapeutic developers of all sizes and at any clinical stage because we have implemented eBRs effectively in collection, manufacturing, and testing processes, and our batch records are even integrated with core quality system modules, so that there is an electronic tie to the records history of document control, the operator training records, any quality events that occur and all relevant equipment and material qualifications.
In effect, a seamless electronic chain of custody is achieved, and complete traceability is safeguarded through the eBR design…. You can learn more about how we implement this system and how it impacts advanced therapies here.
The goal of every biotech company is to translate innovation into impact, delivering life-changing therapies to patients. Achieving that goal requires more than groundbreaking science; it demands efficient, scalable, and reliable manufacturing processes.
Through the early adoption of eBRs, biotech teams can avoid the pitfalls of fragmented records, accelerate their regulatory journey, and position themselves as credible, investment-worthy organizations. And with MasterControl fully integrated into our systems at BBG Advanced Therapies, we’re helping our partners make that leap, without the headaches typically associated with manual or legacy approaches.
If you’re navigating these decisions for your program, we’d love to collaborate. Together, we can design a manufacturing foundation that supports both scientific excellence, collaborative compliance, and long-term business success.
Feel free to connect with me, Adrienne B. Mendoza, MHA through direct messages on LinkedIn, or reach out through our website bbgat.org, using our contact us page.
What are your experiences?
We’ve shared our perspective on the benefits of early eBR adoption, but every biotech’s journey is unique. What questions do you have about electronic batch records, and what advice would you offer to others considering this transition?
Let’s build a valuable resource together in the comments! 💬



