Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on December 31, 2024
Advanced therapies such as CAR T-cell treatments, MSCs, and tissue-based regenerative medicines represent a monumental shift in how we treat challenging conditions. These therapies hold the potential to transform outcomes for patients facing diseases that were once untreatable. But delivering on this promise isn’t easy – it requires navigating what I call the “innovation gap,” the space between scientific breakthroughs and real-world patient access.
Every blueprint starts with an assessment of the “site conditions” – the realities and constraints that shape what can be built. Think about poor site planning: Would you build an office space without considering where the plumbing or power lines connect? In the world of advanced therapies, these site conditions include manufacturing bottlenecks, regulatory hurdles, logistical barriers, and the complex interplay among stakeholders.
In the sections that follow, we’ll envision the ideal healthcare ecosystem for advanced therapies, examine the current patient journey, and identify key risks impeding access. These insights lay the foundation for exploring how we can design bold, collaborative solutions to bridge the gap and bring advanced therapies to the patients who need them most.
Imagining a “Healthy” Ecosystem for Advanced Therapies
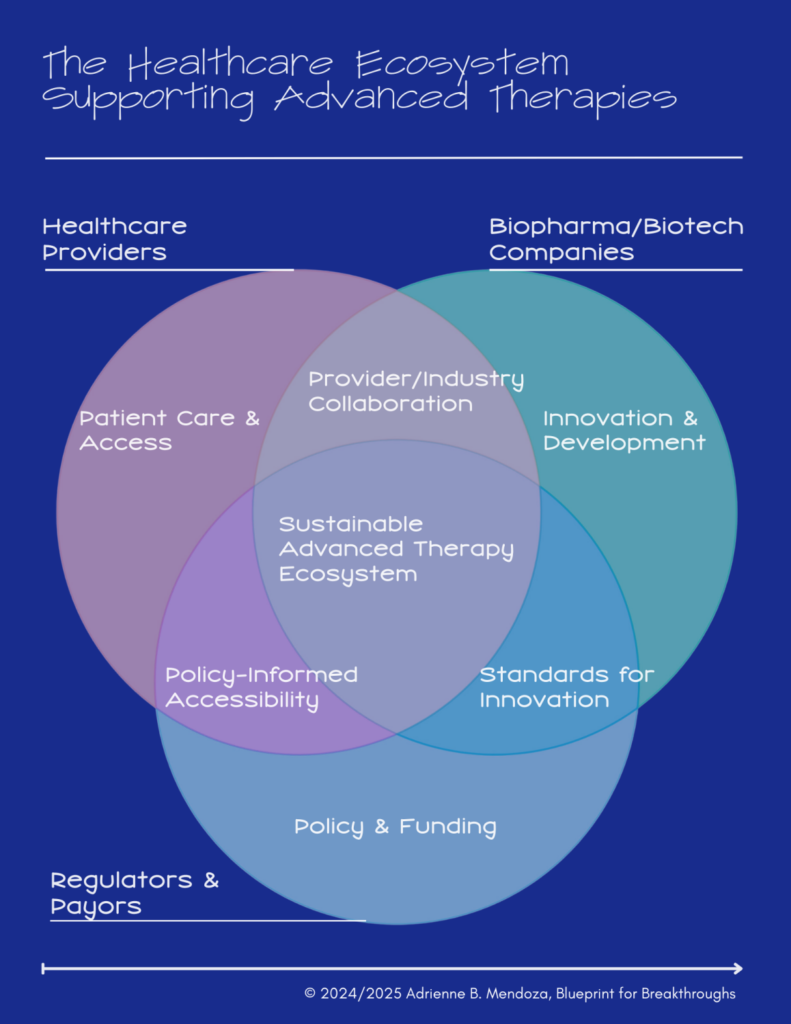
To understand the innovation gap, it’s important to first imagine how an ideal healthcare ecosystem would operate. In a perfect system, Healthcare Providers, Biopharma/Biotech Companies, and Regulators & Payers work together seamlessly, creating a streamlined path from innovation to patient care.
In this ecosystem:
- Healthcare Providers ensure timely access to therapies by identifying eligible patients, coordinating care, and delivering treatment effectively.
- Biopharma/Biotech Companies scale innovation and manufacturing to meet demand while maintaining quality and affordability.
- Regulators & Payers establish frameworks that balance safety, efficacy, and accessibility through streamlined approvals and equitable reimbursement models.
The intersections of these roles, collaboration on policy, aligned standards for innovation, and streamlined processes, form the backbone of a sustainable advanced therapy ecosystem.
The Patient Journey Reality: A Window Into the Innovation Gap
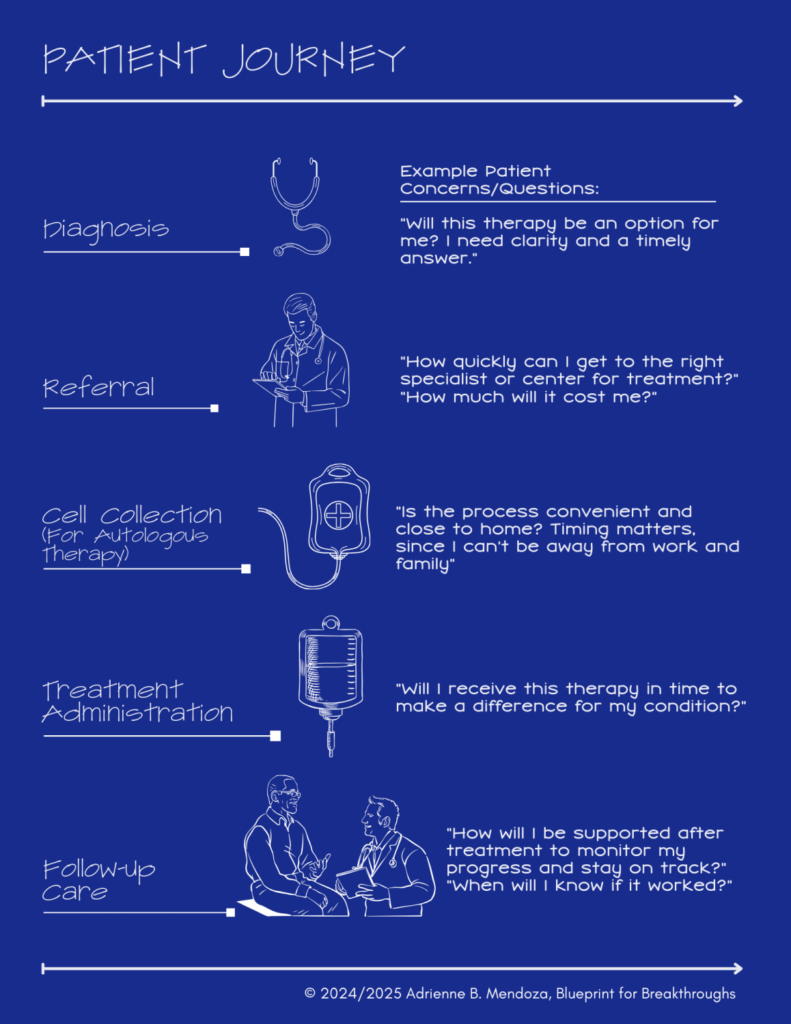
At the heart of this ecosystem is the patient journey – a highly personalized process that highlights systemic gaps creating barriers to access. Many patient questions lack clear answers, underscoring the need for bold, innovative solutions across the ecosystem.
- Diagnosis: Patients face delays and uncertainty about eligibility, as advanced therapies are rarely discussed in care settings, given the standards of care most relied upon.
- Referral: Unclear pathways and geographic disparities typically limit access to a handful of specialized centers.
- Cell Collection: Processes must be timely and convenient for patients balancing health, work, and family (aka: their support system).
- Treatment Administration: Coordination among providers, manufacturers, and centers is crucial to ensure timely delivery, yet these therapies are still new and coordination processes are often underdeveloped.
- Follow-Up Care: Monitoring for efficacy and side effects is critical but often poorly coordinated, leaving patients without adequate support. Follow-up care can also impacted by geographic disparities in access.
Key Risks Impacting Accessibility
In imagining a well functioning healthcare ecosystem earlier, seamless collaboration among healthcare providers, biopharma companies, and regulators could ensure advanced therapies reach patients effectively. However, in reality, significant gaps exist – and its the evidence of the “innovation gap.” Addressing these risks is critical to bridging this gap and ensuring therapies reach the patients who need them.
These risks fall into three categories: high, moderate, and low, each representing a different level of challenge to advancing and scaling therapies.
1. High Risks: The Foundation for Progress
High risks are foundational barriers that prevent advanced therapies from scaling effectively. These risks create bottlenecks that slow progress, increase costs, and restrict patient access.
Examples of High Risks:
- Provider Capacity and Geographic Access: Limited treatment centers and uneven distribution of resources make it difficult for many patients to access therapies, especially in rural or underserved areas.
- Scalability and Manufacturing Challenges: Complex manufacturing processes make scaling production costly and time-intensive.
- Misaligned Reimbursement and Regulatory Frameworks: Therapies face prolonged delays when reimbursement models fail to align with clinical value, or when frameworks struggle to adapt to the nuances of personalized medicine.
Why This Matters: Addressing high-risk challenges is essential to lay the groundwork for accessibility. Without solving these foundational issues, the system cannot function effectively at scale.
2. Moderate Risks: Unlocking Access
Moderate risks stem from inefficiencies and delays in how therapies are delivered. These risks may not halt progress but slow down adoption and limit patient access. For the most part, these are the risks we see presently playing out with commercially approved options that have moved to earlier lines of treatment.
Examples of Moderate Risks:
- Communication Gaps Between Stakeholders: Challenged coordination between providers, manufacturers, and therapy developers can delay patient referrals and disrupt treatment timelines.
- Delays in Regulatory Approvals: Advanced therapies often face long approval processes due to regulatory frameworks that have existed for non-personalized therapies – that haven’t adapted to their complexity.
- Policy Misalignment: Policies that fail to prioritize accessibility, such as unclear referral pathways or restrictive coverage criteria, create unnecessary hurdles for both patients and providers.
Why This Matters: These risks limit how quickly and efficiently therapies can be scaled and delivered. Addressing them requires greater collaboration and innovation across the ecosystem.
3. Low Risks: Refining and Optimizing
Low risks involve fine-tuning processes that, while not critical, can hinder long-term sustainability and efficiency.
Examples of Low Risks:
- Integration Across Stakeholders: Achieving full integration across healthcare providers, biopharma companies, and regulators is aspirational but not immediately necessary for access.
- Non-Critical Operational Gaps: Inefficiencies in areas like post-treatment follow-up or non-essential support functions can slow processes but don’t create significant barriers.
Why This Matters: While low-risk issues don’t require urgent attention, addressing them helps create a more sustainable and efficient system over time.

Designing & Building Systems of the Future
The innovation gap is not an insurmountable challenge. Rather, it’s an opportunity to reimagine the systems and processes delivering advanced therapies to patients. By addressing the key risks and fostering collaboration across the healthcare ecosystem, we can create a future where these transformative treatments are accessible to all who need them.
Several potential solutions can help bridge these gaps and future editions of Blueprint for Breakthroughs will delve deeper into these strategies, and more, offering actionable insights and opportunities for collaboration:
- Scalable Manufacturing: Leveraging automation, single-use bioreactors, and decentralized production hubs to reduce costs and increase efficiency.
- Logistical Innovations: Solutions like mobile leukapheresis (cell collection) teams and streamlined cell processing workflows to expand access and reduce patient burden.
- Provider Training and Support: Equipping healthcare providers with resources to navigate patient selection, logistics, and side effect management.
- Regulatory and Reimbursement Alignment: Developing frameworks that support faster approvals and predictable reimbursement models.
- Advanced Analytical Testing: Real-time potency assays and standardized quality controls to accelerate production without compromising safety.
I’m working with a team of collaborators, actively engaged in advancing many of these solutions. From supporting scalable manufacturing and advancing analytical testing to enabling logistical innovations and fostering partnerships with healthcare providers, we aim to help transform the promise of advanced therapies into real-world patient impact. Feel free to reach out if you’d like to work together.



