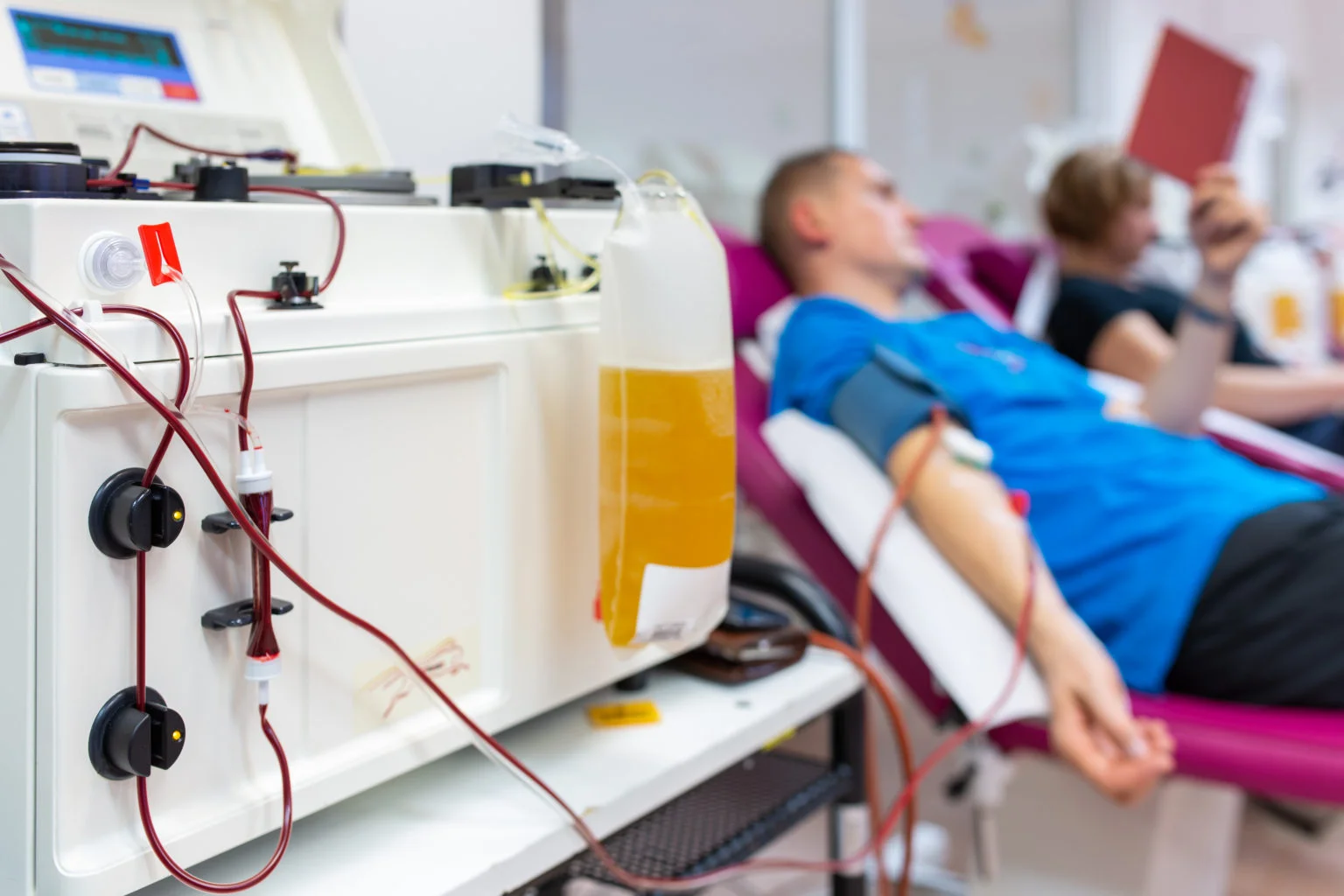
While the process of donating blood and donating plasma is similar, and both blood and plasma centers are closely regulated by the U.S. Food and Drug Administration, there are some significant differences between them:
The first difference
Most blood donors with South Texas Blood & Tissue and other blood centers give whole blood, which is red cells, platelets and plasma, in a single donation.
Plasma donors give just plasma, the liquid that carries the red cells and platelets through the bloodstream. A process called apheresis separates the plasma from the cells, and the cells are returned to the body.
The second difference
The FDA requires collected blood be marked as from either a volunteer donor or a paid donor. U.S. hospitals will not use paid donor blood for liability reasons. As a consequence, there is no reason for blood centers to pay blood donors.
Plasma from paid donors does not have to be labeled because it is not intended for transfusion into patients. Most of it is broken into different products for pharmaceuticals.
The third difference
Plasma separated from red cells and platelets is referred to as “recovered plasma” and can be transfused into patients for therapeutic reasons.
Plasma center collections are referred to as “source plasma” or “plasma for fractionation” and can be used to manufacture what are known as “plasma-derived medicinal products.”
The fourth difference
Whole blood donations undergo 12 tests for blood-borne conditions.
Source plasma for is tested for nine conditions, since the manufacturing of plasma-derived medicinal products clears human T-lymphotropic viruses (HTLV), West Nile virus and the virus that causes Chagas disease.