Blueprint for Breakthroughs is a LinkedIn newsletter published by Adrienne B. Mendoza, MHA, SVP BioBridge Global and Chief Operating Officer (COO), BBG Advanced Therapies
Originally published on LinkedIn on February 18, 2025
Building the Infrastructure is One Step – Getting Buy-In is the Key to Success
One Dilemma – Two Perspectives
Imagine you’re a physician treating a patient who has exhausted conventional options. You’ve heard about a promising cell therapy in clinical trials, but you’re unsure where to refer them, what real-world data supports its use, or how to track their progress if they participate.
Now, picture yourself as a biotech executive bringing that same therapy to market. Your treatment holds immense potential, but trial enrollment is slow because providers hesitate to refer patients due to a lack of long-term data. Regulatory approvals remain uncertain, and every delay shortens the window of commercial exclusivity, reducing the therapy’s value and limiting patient access.
Unlike conventional drugs, advanced therapies require long-term monitoring to assess durability, efficacy, and safety. Yet, there is no standardized system to collect and share this critical real-world data across stakeholders. This gap contributes to the extended timelines for bringing advanced therapies to market – often 10-15 years, compared to 8-12 years for biologics.
A Conversation with Dr. Stephen Sullivan
Over the past few months, we’ve explored the infrastructure challenges slowing the adoption of advanced therapies – Whether it’s referral barriers, logistical roadblocks, or provider hesitancy. Following one of these discussions, Stephen Sullivan, PhD, MBA, FRSM reached out with an idea:
💡 What if another key missing piece is a global patient registry?
Dr. Sullivan, a longtime leader in regenerative medicine, has seen firsthand how the lack of standardized real-world data limits progress. Without a registry, physicians struggle to track patient outcomes, biotech companies face delays in trial enrollment and regulatory approvals, and patterns in treatment effectiveness remain unclear. The result? Patients wait longer for access to life-saving treatments.
While Dr. Sullivan’s expertise is in iPSC-based therapies, he believes the same infrastructure could benefit all advanced therapies. But as we discussed his proposal, one thing became clear: building the infrastructure is just one part of the equation – gaining stakeholder buy-in is the real challenge.
A registry isn’t just a database – it must be a shared resource that benefits all participants. If key players in the healthcare ecosystem don’t see its value, it risks becoming another underutilized system.
A New Application for a Proven Concept
Registries have already proven their value in medicine, particularly in rare diseases and organ transplantation, where they help track long-term outcomes, enhance provider confidence, and streamline regulatory approvals.
While patient registries focus on real-world outcomes, clinical trial registries document interventional research, and cell line registries standardize biological materials. Each plays a distinct role in advancing medical knowledge, supporting regulatory decisions, and improving patient care.
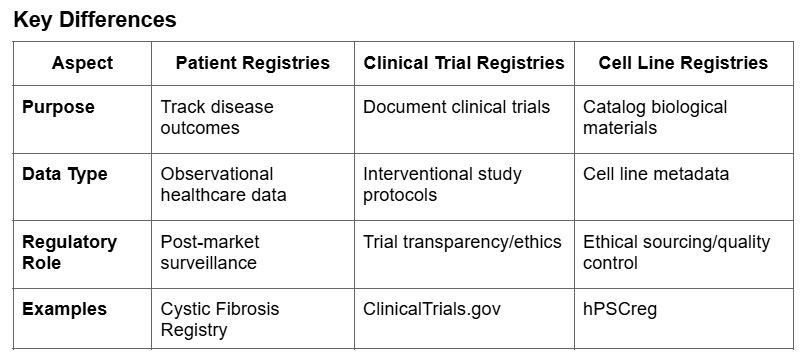
For advanced therapies, a registry must go beyond data collection – it should drive adoption, ensure long-term safety tracking, and build confidence across stakeholders. As Dr. Sullivan explains, a well-structured registry could:
- For physicians: Provide a trusted reference for identifying eligible patients and monitoring outcomes.
- For biotech companies: Reduce trial enrollment delays, strengthen reimbursement cases, and extend commercial viability.
- For regulators: Offer real-world evidence to streamline approvals and post-market surveillance.
- For patients: Allow participation in a system that improves access while contributing to future advancements.
The Business Case for a Global Patient Registry
From an industry perspective, a patient registry isn’t just about data collection – it’s about improving valuation and reducing risk.
“Every month a therapy is delayed in getting to market is lost revenue and a shorter patent lifespan, which devalues the most critical asset to a biotech firm – it’s IP” Dr. Sullivan explains. “If we can use real-world data to streamline regulatory reviews and increase provider confidence in prescribing these therapies post-approval, we create a more efficient and commercially viable pathway.”
For biotech companies developing Advanced therapies, a global registry could:
- Accelerate trial enrollment by reducing provider hesitation.
- Support faster regulatory approvals with real-world safety and efficacy data.
- Extend IP value by maximizing the window of commercial exclusivity.
- Improve investor confidence by demonstrating clear demand and long-term sustainability.
Building a Usable and Scalable Global Registry
Dr. Sullivan outlined key principles for developing a patient registry that is functional, scalable, and widely adopted:
- Usability for Physicians – The registry must integrate into clinical workflows, making it easy for providers to contribute data without adding administrative burden.
- Interoperability – The system must work across institutions and pull data from electronic health records (EHRs).
- Global Collaboration – A registry should facilitate international data-sharing rather than be limited by geography.
- Long-Term Tracking – Given the nature of iPSC therapies, longitudinal data collection is essential for understanding durability and long-term safety.
- Industry & Regulatory Alignment – The registry must provide insights that benefit therapy developers, regulators, and payers, ensuring it drives adoption and accelerates approvals.
- Dynamic Platform & Data Architecture – A well-structured registry with standardized terminology, clear hierarchies, and intuitive analytical tools ensures data is easily accessible, reducing the time and effort needed to identify critical clinical patterns and outcomes.
“Right now, iPSC therapy development is fragmented,” Dr. Sullivan says. “We need a shared infrastructure that allows us to learn from every patient who receives these treatments so we don’t keep starting from scratch with each new trial or product.”
Beyond Infrastructure: What It Takes to Make This Work
Dr. Sullivan emphasizes that building the right infrastructure is only part of the challenge. The real key to success is securing buy-in from key stakeholders: biotech leaders, regulators, physicians, and payers.
To be effective, a global patient registry must:
✔ Be Usable for Physicians – Seamlessly integrate into clinical workflows to encourage participation.
✔ Ensure Interoperability – Work across institutions and pull data from electronic health records (EHRs).
✔ Support Global Collaboration – Facilitate international data-sharing rather than be restricted by geography.
✔ Enable Long-Term Tracking – Capture durability and safety data over extended periods.
✔ Align with Industry & Regulators – Provide insights that benefit therapy developers, regulators, and payers.
✔ Utilize a Dynamic Platform – Ensure standardized terminology and user-friendly analytics to identify clinical patterns efficiently.

Next Steps: Moving from Concept to Action
So, how do we move from an idea to an actionable plan?
Dr. Sullivan is actively bringing this concept to industry meetings and engaging key stakeholders to refine the framework. If you’re involved in cell therapy development, healthcare policy, or investment in advanced therapies, now is the time to shape this initiative.
If you’re interested, reach out to either Stephen Sullivan, PhD, MBA, FRSM, or me, Adrienne B. Mendoza, MHA, through LinkedIn messenger.
👉 What do you think? Could a global patient registry be the missing piece in advanced therapy development?
Let’s Collaborate!
If your organization is developing or delivering Advanced Therapies and needs support with starting materials, leukapheresis services, process and analytical development, cell therapy testing, biomanufacturing, or clinical research, the team at BBG Advanced Therapies is here to help. Let’s work together to scale solutions and ensure these transformative treatments reach more patients, faster. Feel free to reach out to me directly or connect with us through our website, bbgat.org



