Production space is composed of 6,700 square feet, and is designed to be compliant with U.S., European, and Japanese regulations for cell and cell-based biologics.
Cleanrooms are sized for a wide range of production volumes up to 250L scale bioreactors. Equipment in the cleanrooms mirror the equipment available in the Process Development Lab, reducing variability in scale up and cGMP manufacturing for both clinical and commercial production phases.


To support Advanced Therapies manufacturing, our technical capabilities include expertise in isolating peripheral blood mononuclear cells (PBMCs), hematopoietic stem cells (HSCs), T cells, and other specialized cell populations.
We can adapt and tailor procedures to match specific therapy requirements and optimize yield and viability.
The cell manufacturing facility is designed for clinical and commercial cGMP processing. The facilities have four Grade B cell culture processing suites supported by a Grade B purification suite, Grade C solution preparation suite, and a Grade C storage suite with a Grade D glass wash and autoclave suite. All of the suites are connected by a Grade C entry corridor and a Grade D exit corridor, allowing for unidirectional flow of materials and personnel.
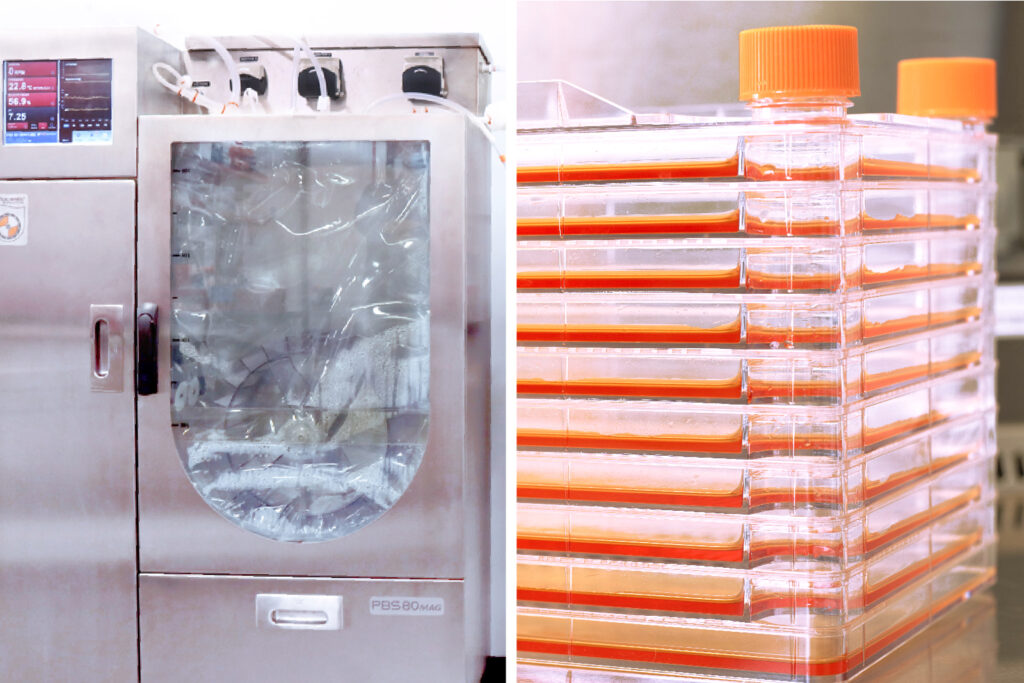
Cell expansion equipment includes:

Harvesting cells or other products is done by centrifugation and filtration techniques. Washing and concentrating cellular derived products is done by ultrafiltration or diafiltration. Tangential flow filtration can be performed from 3L-80L, which scales accordingly with vertical wheel bioreactor systems. Final product filtration can be performed in-line, creating a closed sterile system that is optimized for cell therapy products.
Equipment utilized includes:

Cleanrooms include a Grade B Fill/Finish suite suitable for clinical fills with the flexibility for manual fills or automated fills via the Terumo FiniaTM or Sartorius Fill ItTM.

Multiple LN2 and -80°C freezers allow forflexibility and business continuity. Storage facilities are temperature-monitored and locked with controlled access. LN2 freezers are currently supported by dewars, but construction is underway to provide hard-piped bulk LN2 supply with both types of freezers on a back-up power supply to ensure all products are safely stored.
Scroll through for a virtual tour of the biomanufacturing facility.
