Unique collaboration begins to link the CGT supply chain from end to end to tackle hard scale-up and quality challenges across the industry
Terumo Blood and Cell Technologies, a medical technology company specializing in a portfolio of products, software and services for blood component collection, therapeutic apheresis and cellular technologies, today announces it has signed a new collaborative agreement with GenCure, a subsidiary of BioBridge Global, to extend and unify cell and gene therapy manufacturing solutions.
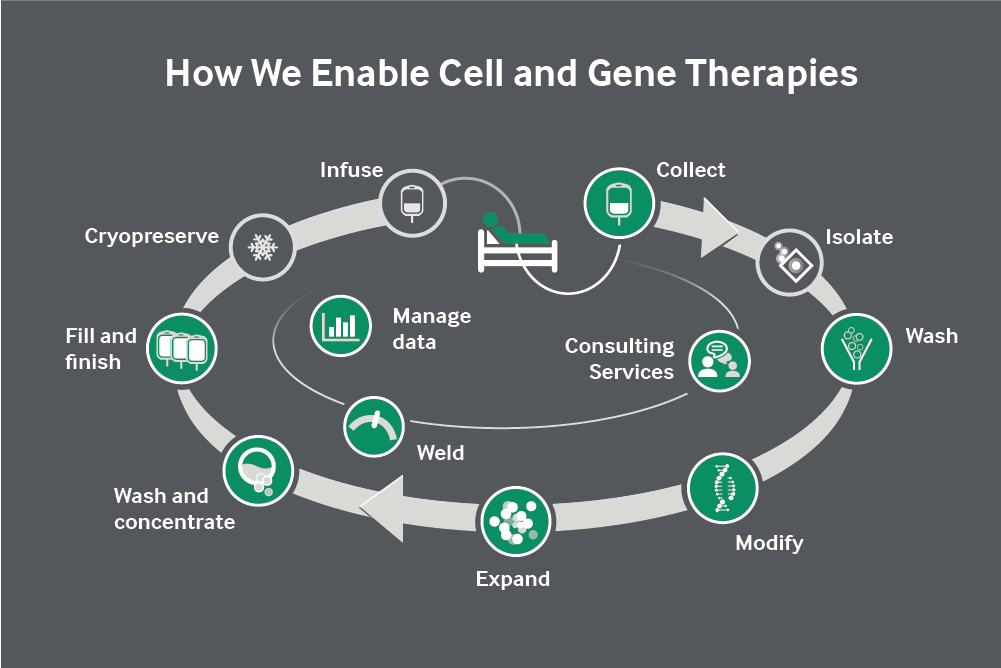
The latest agreement between Terumo Blood and Cell Technologies (Terumo) and BioBridge Global follows a series of long-running activities between the organizations for more than 20 years, including with a number of BioBridge Global’s subsidiaries. It is anticipated that this latest agreement will extend into a broader collaboration with the full array of BioBridge Global’s subsidiaries. Both parties have signed the new agreement to further combine technologies and expertise to cater to the demand across the cell and gene therapy (CGT) industry for smart systems that are automated and closed. These smart-systems allow for real-time response and refinement to the living therapies being produced inside the systems.
This collaboration will initially utilize expertise from Terumo and GenCure to establish a reference manufacturing center for BioBridge Global. This includes both organizations jointly developing core process and manufacturing operations. The reference site will enable GenCure to deliver a full solution for cell and gene therapy developers. It will be based at GenCure’s process development and cGMP manufacturing facility at the VelocityTX campus in San Antonio, Texas, U.S.A.
Terumo will contribute a variety of products from its portfolio to allow in-plant optimization of processes and user feedback, along with investment in new products and process utilization. GenCure will leverage its highly developed expertise in Terumo Blood and Cell Technologies’ cell collection, cell and gene therapy manufacturing and fill-finish equipment. This will significantly enhance its portfolio for developers requiring process optimization and manufacturing support.
The collaboration is scheduled to last three years and is in two phases. The first phase will enable Terumo to select from its portfolio of automated and closed-cell and gene therapy manufacturing devices and share the range of services offered at GenCure. This combination will help solve the critical industry gaps in manufacturing capacity and operational expertise. The second phase will allow GenCure and other BioBridge Global subsidiaries to use Terumo’s data management solutions and value-added services to seamlessly transition between clinical and manufacturing sites by enabling chain-of-custody/identity and consistency of leukapheresis products.
Terumo selected GenCure as a key collaborator that has access under the BioBridge Global umbrella to all of the services needed for cell and gene therapy manufacturing that originates from blood products. These include donor recruitment, cell collections, cell selection and manufacturing facilities and biological testing services. The collaboration allows Terumo to link blood and cell collections with cell and gene therapy manufacturing. There is currently a critical lack of skills and knowledge bridging these areas.
“Despite the rapid evolution of the cell and gene therapy sector, critical challenges such as management between collection, manufacturing sites and logistics still remain unresolved. For over 20 years, Terumo has been working with BioBridge to provide safe, high-quality blood products,” said Delara Motlagh, General Manager, Cell Therapy Technologies, Terumo Blood and Cell Technologies. “We are now combining our product portfolios, expertise and management to provide a vital link between cell collection sites and cell and gene therapy manufacturing sites, while addressing capacity and ensuring GMP production. This will provide a route to deliver cutting-edge cell and gene therapies through pursuing new standards of collection and manufacturing, including quality, consistency and demonstrated scalability.”
Says Martin Landon, Chief Executive Officer of BioBridge Global: “Across the cell and gene therapy sector, collaborations are now proving vital to the rapid development of solutions to a number of key challenges. Our donor-to-patient solutions, which include cell collection, manufacturing and testing, require the best technologies and expertise available in the market. Working with an innovator like Terumo Blood and Cell Technologies will allow us to provide a leading-edge end-to-end solution for cell and gene therapy manufacturing that will be vital to deliver advanced therapies to patients.”